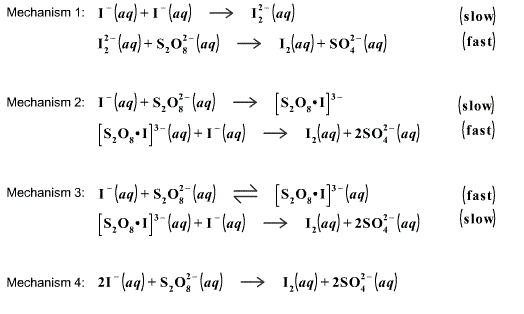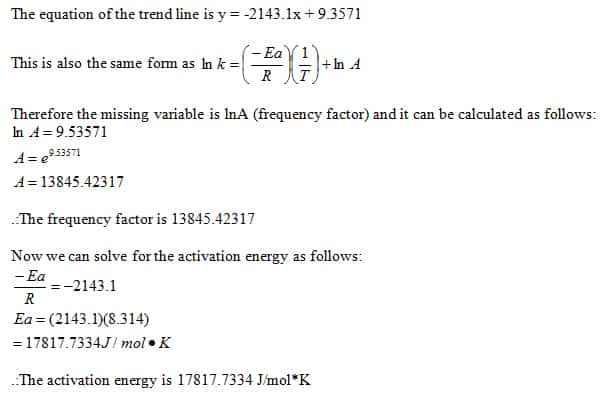162 Lab Iodine Clock Reaction 2014 0227 GF1 - Lab Manual | Clock Reaction Reaction Kinetics: The - Studocu
![SOLVED: The rate law for the iodine clock reaction is given by: Rate = k[IO3- ] [I- ] 2[H+]^2a)This reaction is third order with respect to H+.b)This reaction is first order with SOLVED: The rate law for the iodine clock reaction is given by: Rate = k[IO3- ] [I- ] 2[H+]^2a)This reaction is third order with respect to H+.b)This reaction is first order with](https://cdn.numerade.com/ask_previews/494c958b-2794-4a7f-861f-022f5f445939_large.jpg)
SOLVED: The rate law for the iodine clock reaction is given by: Rate = k[IO3- ] [I- ] 2[H+]^2a)This reaction is third order with respect to H+.b)This reaction is first order with
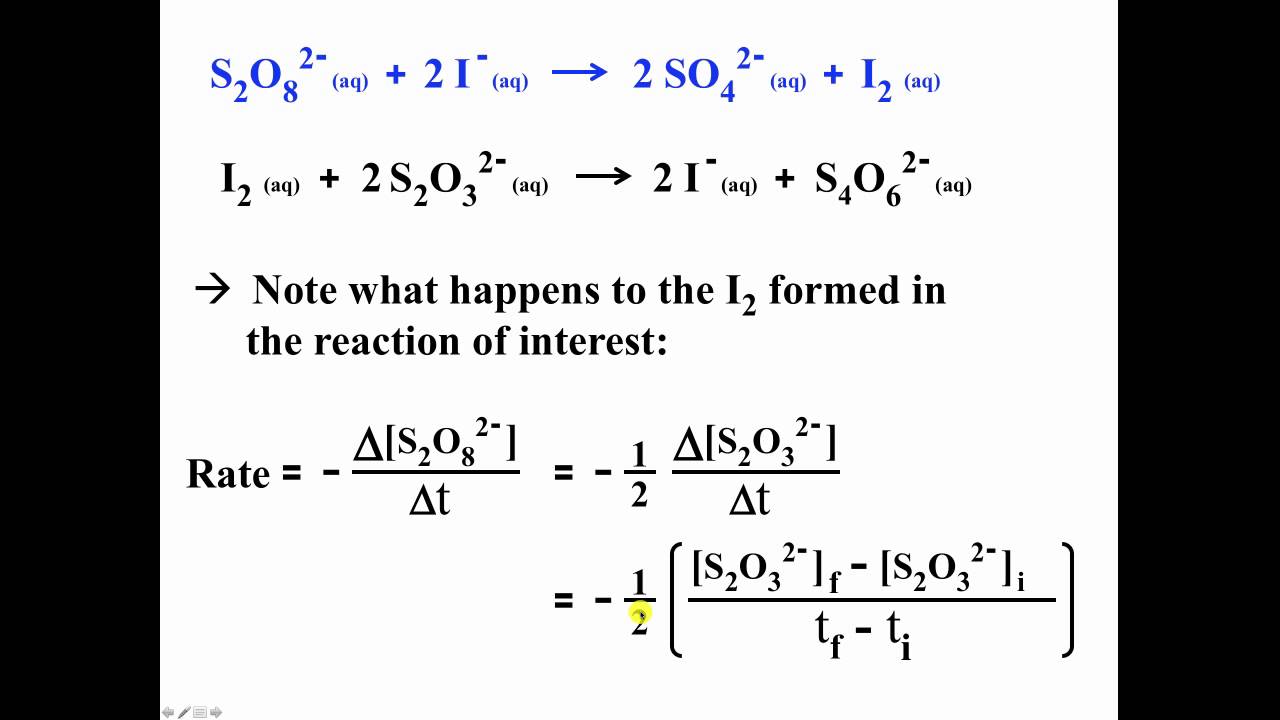
What is the reaction order with respect to [S2O8 2-] and [I-] in a persulfate-iodide reaction? - Quora
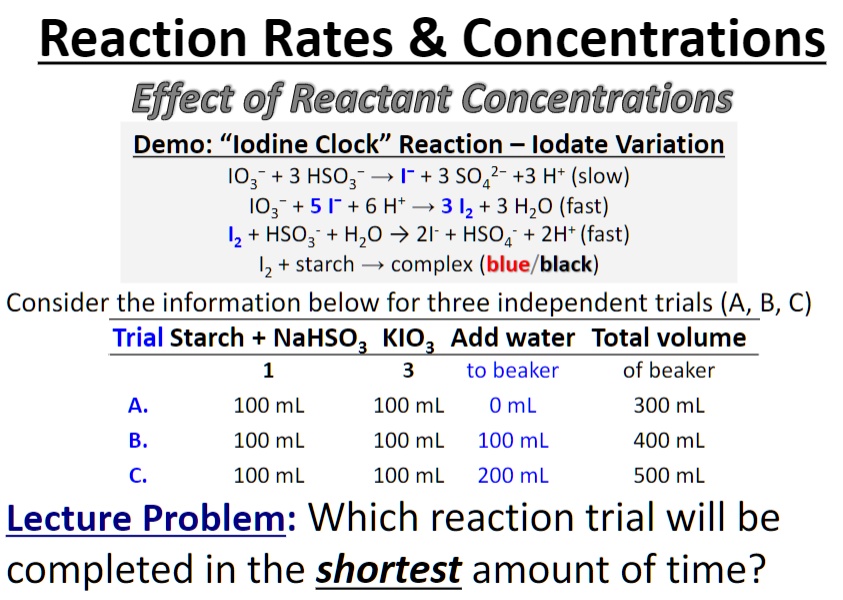
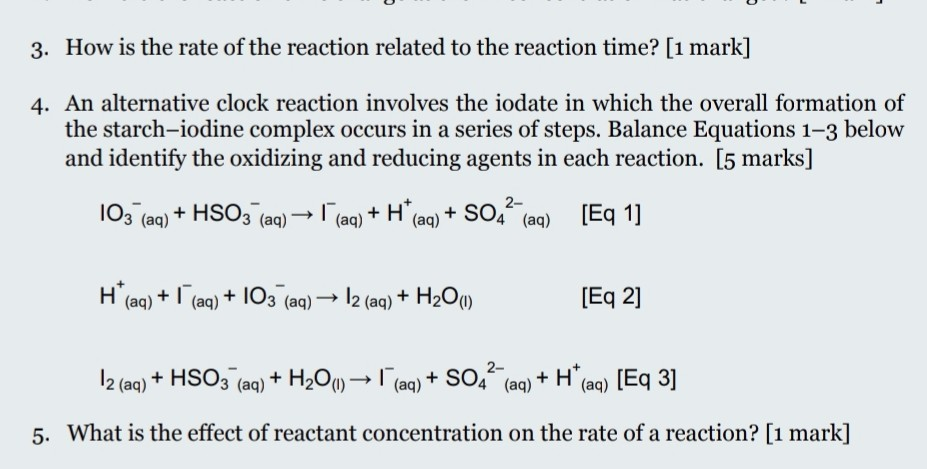
SOLVED: Reaction Rates Concentrations Effect of Reactant Concentrations Demo: "lodine Clock" Reaction lodateVariation I03 + 3 HSO3 > 1-+ 3 S042-+3 Ht (slow) I03 +51 + 6 Ht> 3 12 + 3

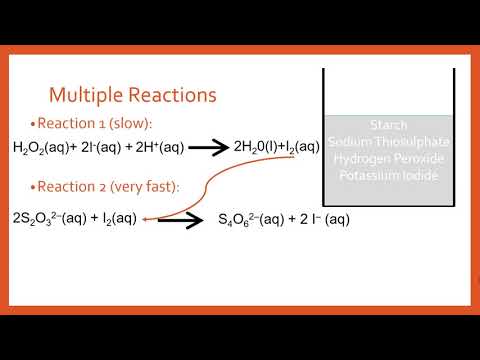
SOLVED: Instructions: Begin by watching this video: Iodine Clock Reaction You will see two different reactions slow reaction in which Iodide ions are produced and fast reaction in which in which the

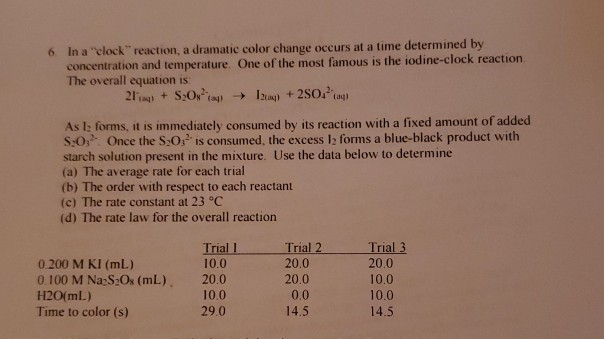
A Closer Examination of the Mechanism of the Hydrogen Peroxide Iodine-Clock Reaction with Respect to the Role of Hypoiodite Species | Journal of Chemical Education

![How to do lab report [Exp 004] Rates of Reaction for Iodine Clock Reaction - YouTube How to do lab report [Exp 004] Rates of Reaction for Iodine Clock Reaction - YouTube](https://i.ytimg.com/vi/L1CtBY_xmZs/maxresdefault.jpg)